Abstract
Introduction: Peripheral T-cell lymphomas (PTCL) are a rare subset of non-Hodgkin lymphomas in which approximately 80% patients will have an overall response to CHOP based therapy but 5-year survival ranges between 20-40%. (Ellin et al Blood 2014) While response by PET/CTs have been helpful in risk stratifying patients (Mehta-Shah Blood Advances 2019), the high rate of relapse after complete response (CR) suggests that more sensitive determinants of minimal residual disease (MRD) could have prognostic and even therapeutic importance. T-cell receptor gene rearrangement sequencing (TCR) is standardly performed by next generation sequencing and is able to detect a known TCR clonotype at 10 -5. (Shah et al AMP 2017) Therefore, in a prospective multi-institutional study, we sought to evaluate the utility of TCR by next generation sequencing in quantifying MRD in PTCL. (NCT03297697). Here we report the results of the TCR evaluation at the end of CHOP-based therapy.
Methods: Subjects with previously untreated PTCL (PTCL-NOS: peripheral T-cell lymphoma, NOS; angioimmunoblastic T-cell lymphoma: AITL; anaplastic large cell lymphoma: ALCL; T-follicular helper phenotype peripheral T-cell lymphoma: PTCL-TFH, monomorphic epitheliotropic intestinal T-cell lymphoma: MEITL) who were being treated with anthracycline based therapy for curative intent were eligible for the study. TCR (TRG or TRB) clonotype was established from baseline formalin fixed paraffin embedded tumor tissue or peripheral blood when tissue samples are not available. TCR clonality was identified using the LymphoTrack® TRG/TRB Assays - MiSeq® (Invivoscribe, San Diego, CA) when top %reads is ≥2.5% and is at least 2x compared to the background.
Results: 43 subjects were enrolled in the study and 41 were evaluable (15 PTCL-NOS, 10 AITL, 7 ALK- ALCL, 5 ALK+ ALCL, 3 PTCL-NOS with TFH phenotype, 1 MEITL). One subject was enrolled with PTCL-NOS and later found to have T-ALL and was withdrawn from the study. One subject with ALK- ALCL withdrew consent prior to sample collection. Subjects initiated treatment with CHOEP (n=16), BV-CHP (n=11), CHOP (n=5), CEOP (n=1) CHOP+azacitidine (n=6), CHOEP+lenalidomide (n=1), EPOCH (n=1). The median age was 65 (range: 22-80). Sixteen underwent a consolidative autologous transplant.
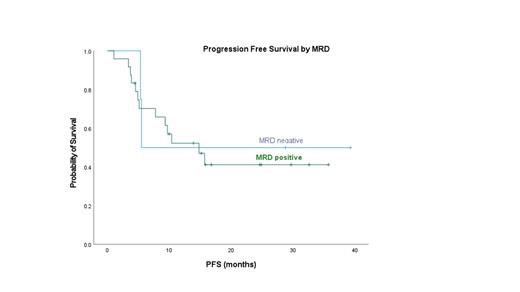
Among the 41 evaluable subjects, 73% (30/41) had tissue samples and 27% (11/41) had only PB samples for TCR clonotype assessment. TCR clonotype was identified in 78% (32/41) tissue samples and not in 23% (9/40: 1 Nodal TFH, 2 AITL, 1 ALK- ALCL, 5 ALK+ ALCL) samples. Of the 32 subjects with baseline clonotype, 2 patients (1 AITL, 1 PTCL) did not have end of treatment (EOT) samples for TCR MRD evaluation. For EOT MRD evaluation, 80% (24/30) were had detectable MRD by TCR (MRD positive) and 20% (6/30) had undetectable TCR (TCR MRD negative). For the EOT evaluation by PET/CT, 63% (19/30) had CR, 10% (3/30) had partial remission (PR) and 27% (8/30) had progressive disease (PD). Among the 6 TCR MRD negative subjects, PET/CT responses were 67% (4/6) for CR and 33% (2/6) for PR, respectively. Among the 24 TCR MRD positive subjects, PET/CT responses were 63% (15/24) for CR, 33% (1/24) for PR and 33% (8/24) for PD. Of those with positive MRD 13/24 (54%) have relapsed/progressed including 8 with primary refractory disease. At the end of CHOP-based therapy, 79% (15/19) subjects with PET CR were TCR MRD positive, and 21% (4/19) were TCR MRD negative. Among the 11 subjects with either PD or PR by PET/CT, 82% (9/11) were TCR MRD positive, and 18% (2/11) were TCR MRD negative. At a median follow up of 20.7 months, progression free survival and overall survival at 18 months did not differ between those who were TCR MRD positive or negative respectively at EOT (OS 64% vs 67% p=0.63; PFS 41% vs 50% p=0.40). We plan to present additional follow up for all patients on the study including MRD post autologous transplant at the time of the meeting.
Conclusions: Measurement of peripheral blood TCR at the end of treatment is feasible in peripheral T-cell lymphomas using next generation sequencing with a known tumor clonotype. Detectable TCR at the end of treatment correlates with lack of CR but the majority of patients in complete remission by PET/CT have a detectable TCR clonotype at end of treatment. Longer follow up is required to determine if consolidative transplant alters TCR dynamics.
This study was supported by the Lymphoma Research Foundation and T-cell Leukemia/Lymphoma Society.
Mehta-Shah: C4 Therapeutics: Consultancy; Ono Pharmaceuticals: Consultancy; Secura Bio: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; Innate Pharmaceuticals: Research Funding; Roche/Genentech: Research Funding; Corvus Pharmaceuticals: Research Funding; Karyopharm: Consultancy; Kiowa Hakko Kirin: Consultancy; Verastem: Research Funding. Jacobsen: Takeda: Consultancy; Syros: Consultancy; Janssen: Research Funding; Novartis: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding. Fehniger: Wugen: Consultancy, Current equity holder in publicly-traded company, Patents & Royalties: related to memory like NK cells, Research Funding; Affimed: Research Funding; Compass Therapeutics: Research Funding; HCW Biologics: Research Funding; Kiadis: Other; ImmunityBio: Research Funding; OrcaBio: Other; Indapta: Other. Kahl: AbbVie, Acerta, ADCT, AstraZeneca, BeiGene, Genentech: Research Funding; AbbVie, Adaptive, ADCT, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva: Consultancy. Bartlett: Celgene: Research Funding; Bristol Myers Squibb: Research Funding; Forty Seven: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Merck: Research Funding; Millennium: Research Funding; Pharmacyclics: Research Funding; Autolus: Research Funding; Seagen: Consultancy, Research Funding; Roche/Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Ghobadi: Amgen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Atara Biotherapeutics: Consultancy; Wugen: Consultancy; Celgene: Consultancy. Moskowitz: Seattle Genetics: Consultancy, Research Funding; Imbrium Therapeutics L.P./Purdue: Consultancy; Merck: Consultancy, Research Funding; ADC Therapeutics: Research Funding; Miragen: Research Funding; Beigene: Research Funding; Bristol-Myers Squibb: Research Funding; Takeda: Consultancy; Incyte: Research Funding; Janpix Ltd.: Consultancy. Jacobsen: Invivoscribe: Current Employment. Olson: Invivoscribe: Current Employment. Vigil: Invivoscribe: Current Employment. Hill: Invivoscribe: Current Employment. Elias: Invivoscribe: Current Employment. Huang: Invivoscribe: Current Employment. Horwitz: ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Affimed: Research Funding; Celgene: Research Funding; Aileron: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal